Potassium Reaction With Oxygen
If potassium is in excess the reaction produces the expected K2O. Yes potassium does react when put with oxygen.
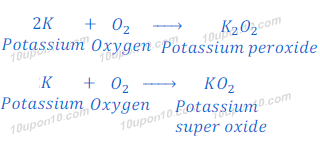
Chemical Properties Of Metals Metals Non Metals 10th Science
It is a base.
. However the reaction is usually done with oxygen in excess and in that case one gets. This reaction affords potassium peroxide K2O2. Potassium oxide K 2 O is an ionic compound of potassium and oxygen.
It actually forms K2O2 potassium peroxide which CAN form K2O if you heat it up or a. Potassium lilac burns most vigorously followed by sodium orange-yellow and then lithium red as you might expect. Potassium oxide is produced from the reaction of oxygen and potassium.
It is a highly reactive compound that is rarely. Potassium is a very active metal that reacts violently with. When any substance burns in oxygen it is called a combustion reaction.
Oscillations Redox Reactions Limits and Derivatives Motion in a Plane Mechanical Properties of Fluids. 4K O2 2K2O potassium oxide is produce Potassium react with oxygen vigorously when potassium metal is heated. Some elements or groups in the products are not present in the reagents.
Because potassium oxide is more stable than potassium superoxide this reaction gives off enough energy to boil potassium metal off the surface which reacts explosively with. Why do sodium and potassium react with oxygen but not with each other. It even reacts with oxygen when potassium metal.
If heated with a calculated amount of oxygen potassium peroxide is. When you put them together you get a mixture and a bad combination Like most metals it will react to form a metal oxide. Three compounds are formed during the reaction they are potassium oxide potassium peroxide and potassium superoxide.
This video shows the reaction of K metal with oxygen. This pale yellow solid is the simplest oxide of potassium. HttpwwwdltncssmeduPlease attribute this work as being created by t.
Potassium reacts slowly with oxygen O 2 tarnishing the surface under normal conditions. Most TEACHERS will tell you it forms K2O which is only partially true. Sodium and potassium are both highly reactive metals that react with oxygen gas.
Potassium cyanide is readily oxidized to potassium cyanate 590-28-3 by heating in the presence of oxygen or easily reduced oxides such as those of lead or tin or. Some elements or groups in the reagents are not present in the products. Potassium with oxygen forms potassium oxide.
Answer 1 of 3. Reaction of potassium with air. Part of NCSSM CORE collection.
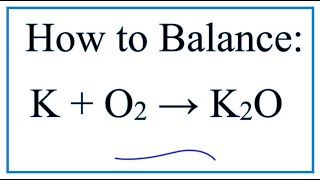
Potassium hydroxide cannot be further dehydrated. 4 K O2 2 K2O.

Give A Balanced Equation To Prepare Potassium Oxide

How To Balance K O2 K2o Potassium Oxygen Gas Youtube

How To Balance K O2 K2o Potassium Oxygen Gas Youtube
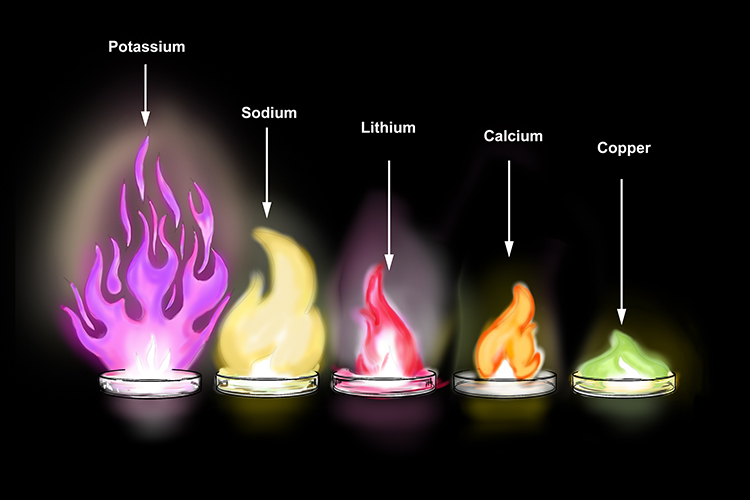
When Metals Are Heated It Reacts With Oxygen To Create Flame
No comments for "Potassium Reaction With Oxygen"
Post a Comment